United States President Joe Biden was tested positive for Covid-19 on Saturday (July 30), immediately after repeatedly testing negatively this week. Doctor Biden, Kevin O’Connor, said that after testing negatively on Tuesday night, Wednesday morning, Thursday morning, and Friday morning, the president was tested positively on Saturday morning in an antigen test. He has no symptoms and feels “pretty good”.
The return of the President’s infection is another case of what is described as “rebound paxlovid” – the phenomenon of patients who have been given antivirus paxlovid drugs, experiencing a return of infection days after negative testing. Before Biden, Dr. Anthony Fauci, the head of the medical officer for the President, experienced a rebound Paxlovid as well.
More than a third of Americans who have been positively tested for Covid-19 this summer have been given Paxlovid, and a large number of patients have been tested back immediately after testing negatively.
Although there is no data on the frequency of infectious recurrence or long -term effects on patients, “rebound paxlovid” is now recognized as something in battle more than two and a half years against the corona virus. In May, the US Public Health Agency, the Center for Disease Control and Prevention (CDC), said that patients who had completed the Paxlovid drug course could be declared positive again, and that these people had to isolate themselves for five days.
What causes a rebound infection?
The fact is this: Biden and Fauci are parents – each 79 and 81 – and both are completely vaccinated. Biden is likely to be infected with the BA.5 sub -variant of Omicron, said Dr. O’Connor last week. BA.5 is the dominant variant in the US today. This is also the most transmitted variant of Coronavirus which has been identified so far. BA.5 has shown a strong ability to avoid the protection of the immune system both protection given by the vaccine, and by the previous Covid-19 infection.
Many doctors and experts are not enthusiastic about paxlovid, especially in cases of vaccinated patients. While Paxlovid has been hypnotized as a “magic drug” against Covid, its success has been discussed especially in the context of drug receiving that is not vaccinated. On April 22, the World Health Organization (WHO) said Paxlovid was “highly recommended”-but for patients with Covid-19 non-bodies who were the highest risk of experiencing severe diseases and inpatients, such as patients who were not vaccinated, older, or immunosuppression pressed.
A report in Atlantic on Saturday quoted Dr. Reshma Ramachandran, a researcher in Yale, said he felt “resignation” about Paxlovid because even though the drug was one of the few Covid-19 treatments that he could offer, he could not say with confidence that the pill will help someone who has been immunized. The same report quoted Bob Wachter, Chair of Medical at UC San Francisco, said that assessing the value of Paxlovid for this patient was “a very complicated three -dimensional chess game.”
The report noted that Biden had begun in Paxlovid immediately after the infection was confirmed, and that in June, Fauci did not take one, but two drug programs.
Okay, so what is Paxlovid, and how does it work?
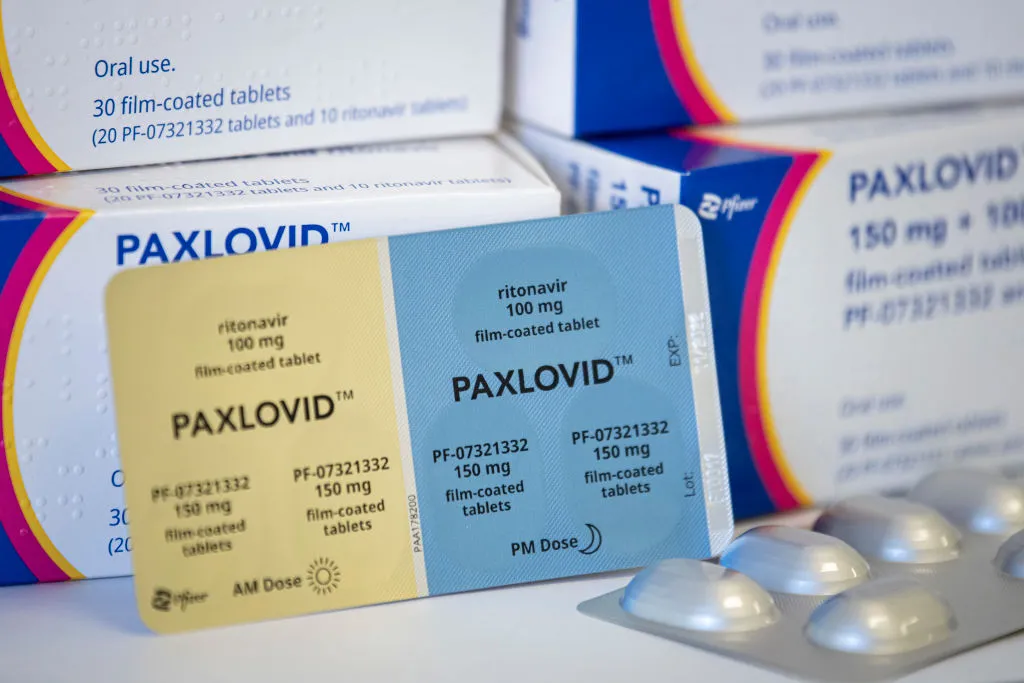
Paxlovid is an antivirus drug developed by Pfizer, which consists of nyrmatrelvir tablets and ritonavir tablets, packed together for oral use. It was given an authorization of emergency use (EUA) by USFDA in December last year.
Paxlovid was given as three tablets – two of the nyrmatrelvir and one of the ritonavir – taken together twice a day for five days. That is, a total of 30 tablets. Paxlovid’s official usfda to be used for only five consecutive days. (After the rebound case in the US, there are some discussions now about whether five days of too little to effectively handle infection, but there is no strong medical opinion about this aspect.)
Of the two components of paxlovid, nymatrelvir inhibits the enzyme virus called protease needed for viruses to replicate itself in the host cell. And the second component, ritonavir, slows down the damage of non -matrelvir to help it remain in the body longer at a higher concentration.
Drugs such as nhythmatrelvir are considered to have advantages over vaccines because they attack vulnerability in viruses that are not mutated like protein spikes – which are targeted at the vaccine – do. As a result, the medicine looks effective against all variants. This is considered very important because the omicron waves have shown that in a large number of cases, vaccines cannot prevent infection, even though they do prevent serious illness and death. (This pattern is also seen in India.)
Besides Paxlovid, the second oral covid-19 drug, Molnupaivir, produced by Merck and Ridgeback, also received FDA authorization in December last year. But it shows a somewhat lower efficacy in clinical trials.
WHO issued strong support from drugs in April this year based on new data from two random controlled trials involving 3,078 patients. Data shows that the risk of inpatients is reduced by 85% after this treatment, WHO said in a statement at that time. In high -risk groups (more than 10% of inpatient risk), that means 84 less inpatient per 1,000 patients.
EUA USFDA for paxlovid is based on clinical data that shows a reduced risk of inpatients or death by 89 percent within three days after the emergence of symptoms, and 88 percent within five days after symptoms, compared to the placebo group.
European Medicines Agency (EMA) issued a suggestion that Paxlovid can be used to care for adults with Covid-19 that does not require additional oxygen and which is at higher risk of severe disease.
Is Paxlovid widely available and prescribed?
In the US, yes. Literally tens of thousands of Paxlovid recipes are written every day. US government data shows more than 5.6 million drug courses ordered, and more than 3 million courses are managed between mid -December 2021 and July 24, 2022. Biden administration has aimed at expanding access to oral antivirus care such as Paxlovid.
On November 16 last year, Pfizer announced that it had signed a voluntary license agreement for Paxlovid which would facilitate the production and distribution of drugs by providing sub-section to generic drug producers that met the requirements.
The Pfizer license agreement with a public health organization supported by the United Nations Patent Patent Health Medicines (MPP) is intended to allow the supply of drugs to 95 low and secondary countries including India, which consists of about 53% of the world population.
It was announced at the time that Pfizer would not receive royalties about sales in low-income countries, and that royalties would be eliminated on sales in all countries covered by agreements during Covid-19 remaining classified as public health emergencies of international concern by whom.
Furthermore, on March 17 this year, MPP announced that they had signed an agreement with 35 companies to produce generic versions of Nymatrelvir, which in a combination with low doses of Ritonavir could be supplied in 95 low and medium income countries. Six companies will produce drug substances, nine will produce drug products, and the rest will do both, MPP said in the release.
These companies are located, besides India, in Bangladesh, Brazil, China, Dominican Republic, Jordan, Israel, Mexico, Pakistan, Serbia, the Republic of Korea, and Vietnam.
MPP, which was founded by United-based United, works to increase access to, and facilitate development, medicines that save lives for low and secondary income countries. MPP partners with civil society, government, international organizations, industry, patients’ groups, and other stakeholders to prioritize and license the medicines needed and gather intellectual property to encourage the creation of generics and the development of new formulations.
So, is the drug used to treat Covid-19 in India?
Although approved in India, the doctor does not prescribe paxlovid widely. Most of the Covid-19 cases in India are mild, and there has been no encouragement from the government for the use of drugs so far.
Nineteen of 35 companies by which MPP has signed a sub-line agreement is India, and they include drug makers such as BioCon Ltd-based in Bengaluru; Glenmark Pharmaceuticals, Sun Pharmaceuticals, and Cipla based in Mumbai; Ahmedabad headquartered Torrent Pharmaceuticals and Cadila Pharmaceuticals; Hetero and Laurus Labs drugs from Hyderabad; and Emcure Pharmaceuticals of Pune.
In April 22’s statement, which said “very concerned” that low and middle income countries can be pushed into “end queues” while accessing Paxlovid’s care in the same way as suffered by these countries when the COVID-19 vaccine supply comes. It is said that the PFizer license agreement with MPP limits the number of countries that can benefit from generic drug production.